Contact : Catherine Debiemme-Chouvy
Principe
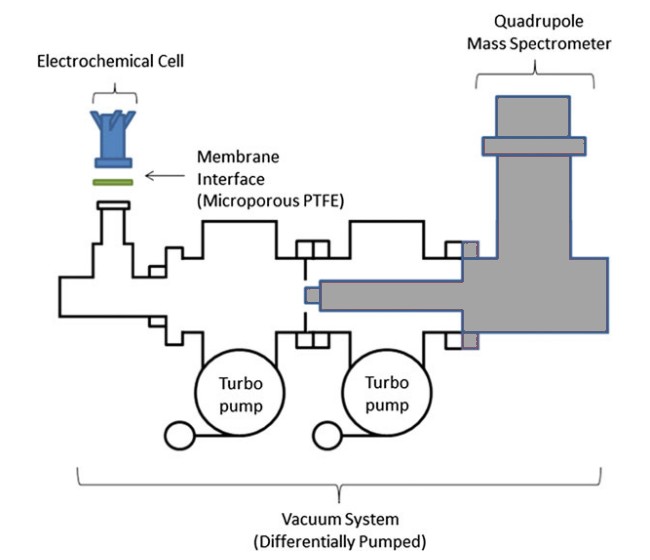
The coupling between an electrochemical cell and a mass spectrometer allows the detection, identification and time-based observation of the gaseous products formed at the working electrode.
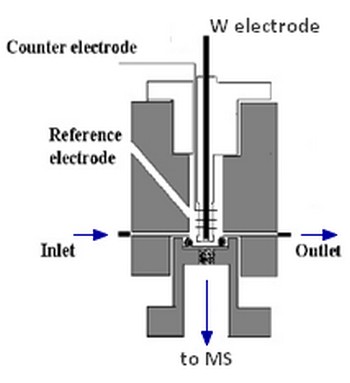
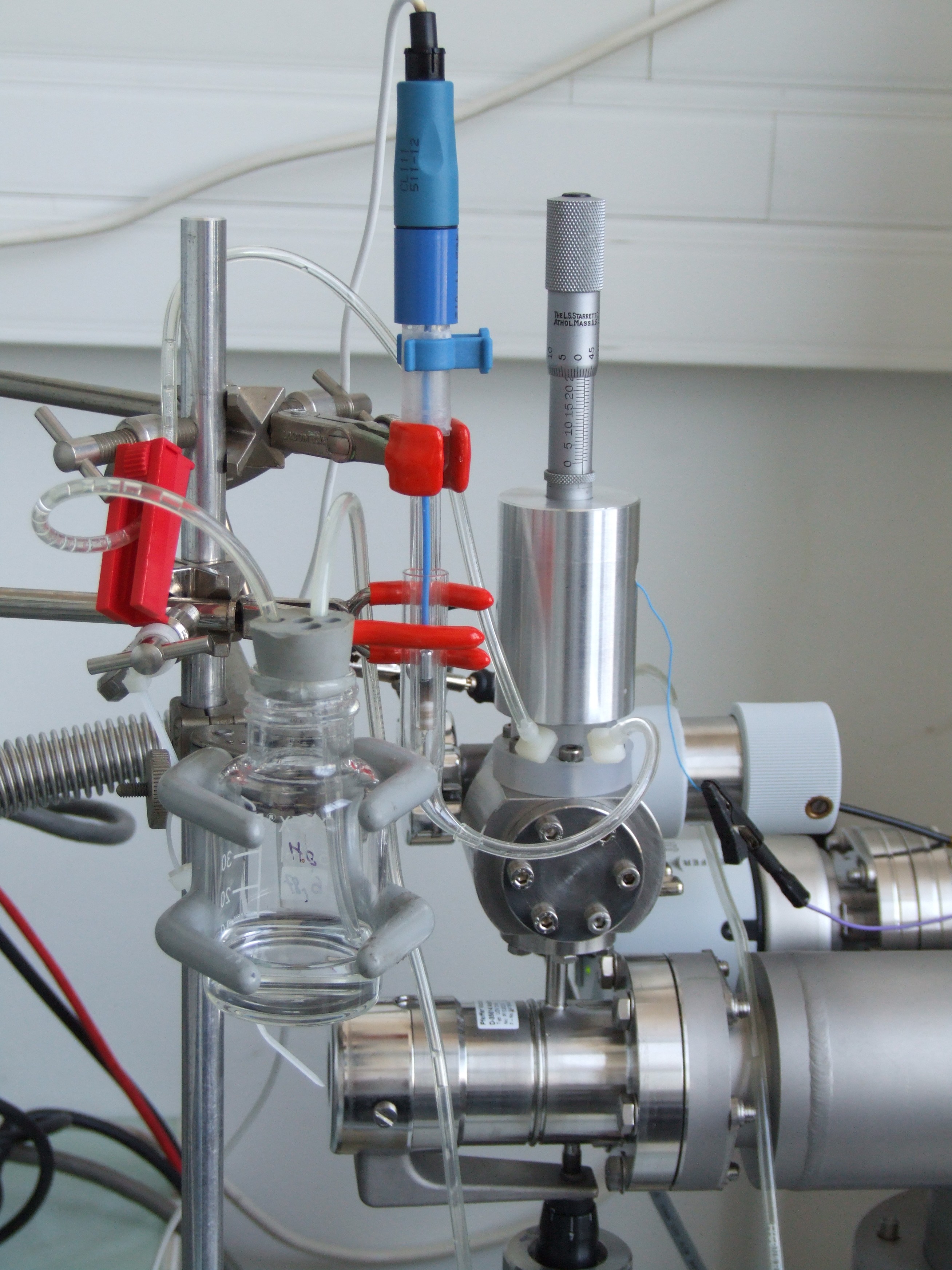
The experimental set-up consists of a three-electrode electrochemical cell connected to a mass spectrometer (quadrupole, m/q < 200), the separation between these two elements being ensured in particular by a hydrophobic microporous membrane in PTFE.

Some examples of application:
a) Methanol oxidation: CH3OH + H2O --> CO2 + 6 H+ + 6 e-
b) "Negative Difference Effect" at a Mg électrode dihydrogen production takes place at open circuit potential or under an ANODIC polarization.
Mg --> Mg+ + 1 e- puis 2 Mg+ + 2 H2O --> 2 Mg(II) + H2 + 2 OH-
c) Nitrite reduction in an acidic medium
- HNO2 disproportionation (pKa = 3,3) : 3 HNO2 --> NO3- + 2 NO + H+ + H2O
- HNO2 reduction by SiMo12O406- : 2 HNO2 + 2 SiMo12O406- + 4 H+ --> N2O + 2 SiMo12O404- + 3 H2O
- HNO2 reduction by SiMo12O406- : 2 HNO2 + 2 SiMo12O406- + 4 H+ --> N2O + 2 SiMo12O404- + 3 H2O
References:
| [1] | H. Baltruschat, Differential Electrochemical Mass Spectrometry, J. Am. Soc. for Mass Spectrometry 15 (2004) 1693-1706. | ||||||
| [2] | N. Fujiwara, KA Friedrich, U. Stimming, Ethanol oxidation on PtRu electrodes studied by differential electrochemical mass spectrometry, J. Electroanal. Chem. 472 (1999) 120–125. | ||||||
| [3] | C. Debiemme-Chouvy, H. Cachet, G. Folcher, C. Deslouis, Electrocatalytic reduction of HNO2 by a silicomolybdate polyanion: a differential electrochemical mass spectrometry study, Electroanalysis 19 (2007) 259-262. | ||||||
| [4] | H. Wang, H. Baltruschat, DEMS study on methanol oxidation at poly- and monocrystalline platinum electrodes: the effect of anion, temperature, surface structure, ru adatom, and potential, J. Phys. Chem. C 111 (2007) 7038–7048. | ||||||
| [5] | L. Jiang, L. Colmenares, Z. Jusys, G.Q. Sunb, R.J. Behm, Ethanol electrooxidation on novel carbon supported Pt/SnOx/C catalysts with varied Pt : Sn ratio, Electrochim. Acta 53 (2007) 377–389. | ||||||
| [6] | A. Stefanova, S. Ayata, A. Erem, S. Ernst, H. Baltruschat, Mechanistic studies on boron-doped diamond: Oxidation of small organic molecules, Electrochim. Acta 110 (2013) 560-569. | ||||||