Contact: H. Perrot (hubert.perrot@sorbonne-universite.fr)
The quartz microbalance is a piezoelectric transducer increasingly used in the field of electrochemistry. The first tests in liquid media were carried out in the early 1980s. It makes it possible to transform a mass variation into a frequency variation, under certain conditions, easily measurable. The great interest of this device is based on its high sensitivity to study in situ and in real time an electrochemical process.
The measuring principle uses techniques such as chronometry: a resonator, usually a quartz crystal, is inserted into an electronic circuit that delivers a very stable signal over time, the whole forming an oscillator. Any disturbance on the crystal surface immediately affects the measured oscillation frequency and a simple Sauerbrey relationship can be established between the mass variation and this frequency1:
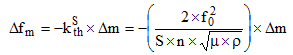
where ∆fm is the microbalance frequency change (Hz), S is the electrode area given by the metallic films deposited on the two quartz sides (cm2), ρ is the quartz density (2.648 g cm-3), μ is the quartz shear modulus (2.947´1011 g s-2 cm-1), n is the harmonic number and f0 is the microbalance frequency in air. With a microbalance working at 9 MHz, a frequency change of 1 Hz corresponds to a mass change of 1.1 ng for a 0.2 cm2 active surface, which represents less than one monolayer of adsorbed oxygen on this surface.
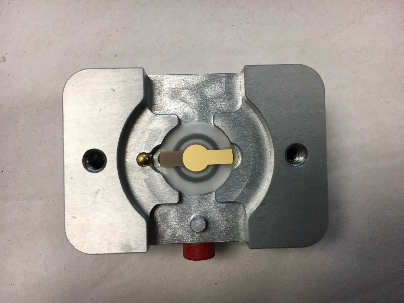
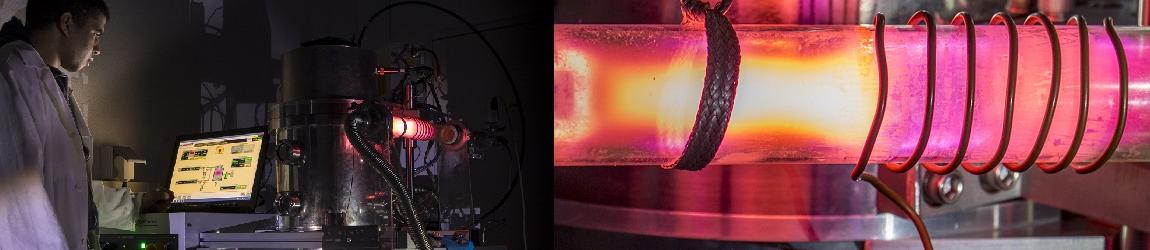
9MHz quartz resonator placed in an AWS cell (AWS company-Spain) |
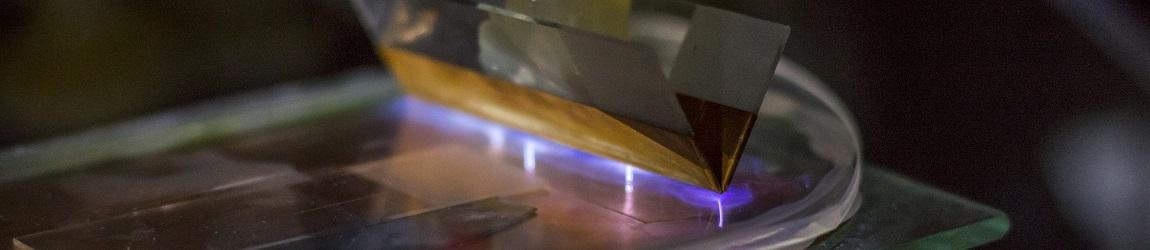
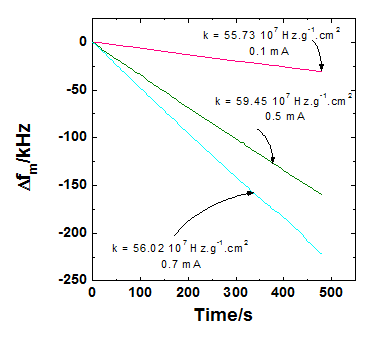
Calibration curves of a quartz crystal microbalance operating at 27 MHz by copper electrogeneration. |
This very sensitive tool has been perfected in the laboratory in order to obtain preponderant information on different electrochemical processes (corrosion2, energy3), biology (ADN sensor4) et physico-chimistry (scaling films5).
References:
1. H. Perrot, “Chemical Sensors and Biosensors", edited by R. Lalauze, ISTE, Wiley 2012, ISBN 978-1-84821-403-3.
2. "Corrosion inhibition of Al 2024 alloy in a dilute NaCl by 1-pyrrolidine dithiocarbamate" , W. Qafsaoui, M.W. Kendig, H. Perrot, F. Pillier and H. Takenouti, Corrosion Science, 92 (2015) 245–255.
3. "Gravimetric and Dynamic Deconvolution of Global EQCM Response of Carbon Nanotube Based Electrodes by ac-Electrogravimetry”, F. Escobar, A. Arnau, J. V. Garcia, Y. Jiménez, H. Perrot and O. Sel, Electrochem. Com., 70 (2016) 73-77.
4. "Development of a mass sensitive quartz crystal microbalance (QCM)-based DNA biosensor using a 50MHz electronic oscillator circuit", G. García-Martinez, E. Bustabad, H. Perrot, C. Gabrielli, B. Bucur, M. Lazerges, D. Rose, L. Rodriguez-Pardo, J. Fariña,C. Compère and A. Arnau-Vives, Sensors, 11, 8 (2011) 7656-7664.
5. "Direct detection of Calcium Carbonate Scaling via a Pre-Calcified Sensitive Area of a Quartz Crystal Microbalance", Y. Chao, O. Horner, F. Hui, J. Lédion and H. Perrot, Desalination, 352 (2014) 103-108.