Laure Fillaud, Emmanuel Maisonhaute
Cyclic voltammetry (CV) is the most often used technique in electrochemistry because it allows to visualize directly the reactivity of systems present or created in the solution or adsorbed at the electrode. The important parameter in CV is the scan rate
Therefore, to access to fast kinetics, one seeks to increase
More specifically, we work in the field of molecular electronics, and the two examples presented below reflect our major activity:
Self-assembled monolayers
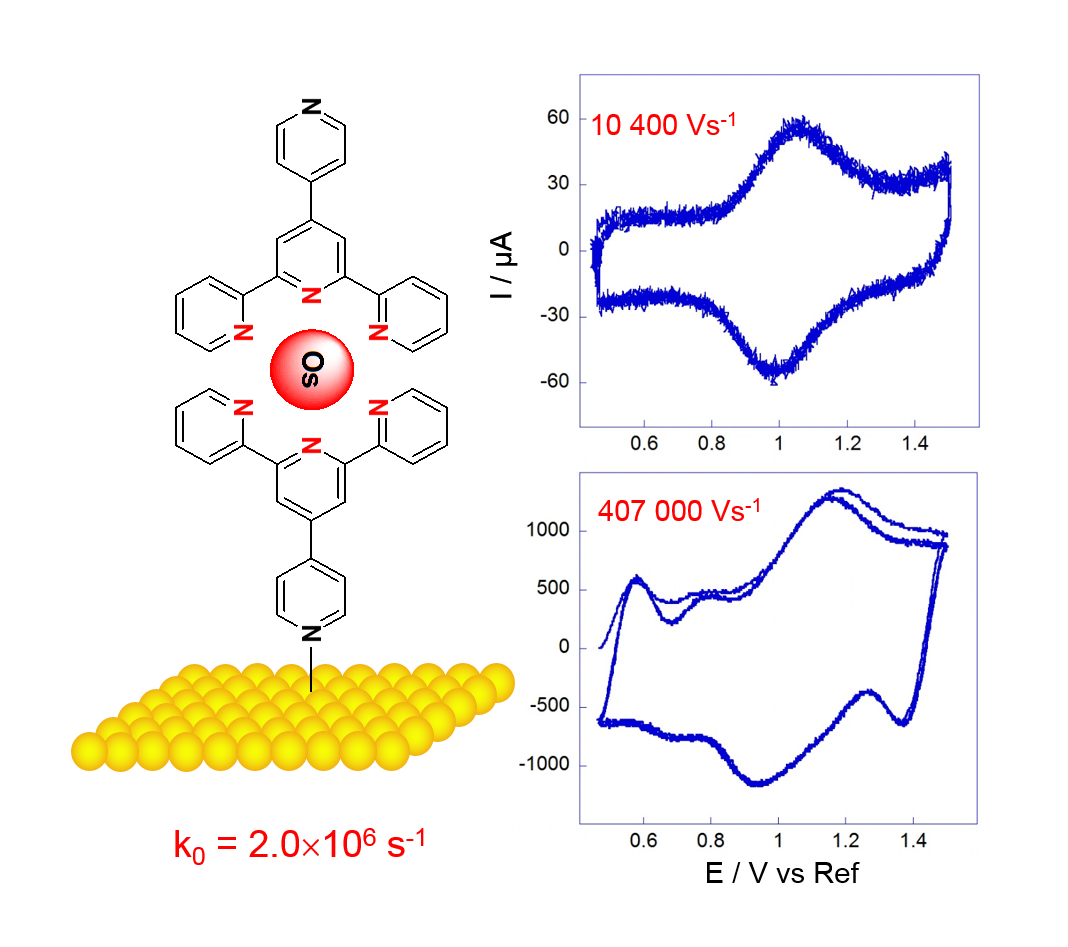
We measure the rate of electron transfer (k0) of a large number of systems, and more specifically of electroactive self-assembled monolayers. When the bridge between the redox center and the electrode is conjugated, electronic coupling is very efficient and
The figure presents the response in ultrafast CV in an electroactive osmium complex. When
Cascades of electron transfers in supramolecular nanoobjects
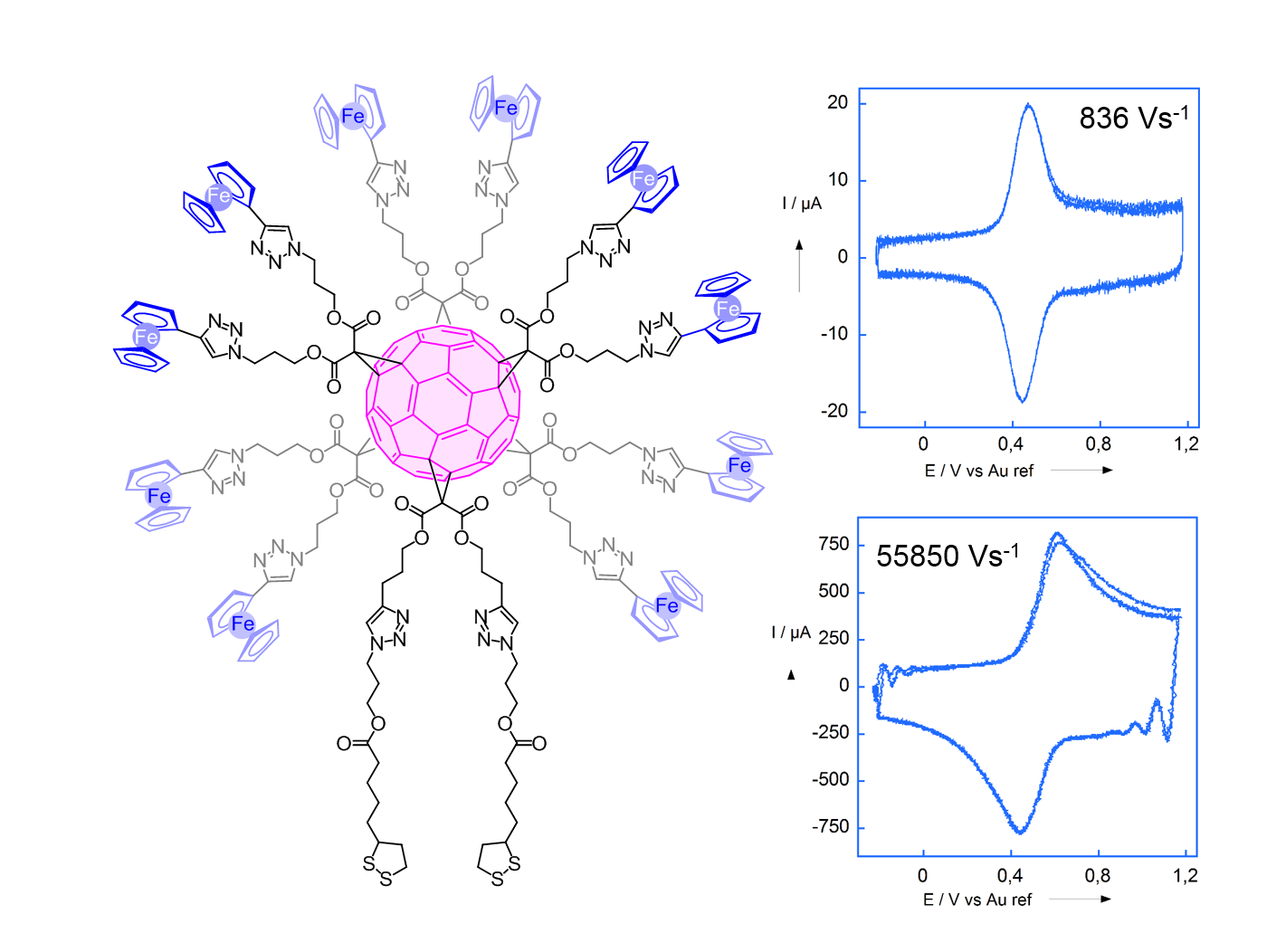 Here, increasing
Here, increasing
The figure presents a redox dendrimer and two CVs obtained onto a gold ultramicroelectrode modified with this dendrimer. When the scan rate increases, the peak shape is modified because only the the redox centers close to the electrode feel the electrochemical perturbation. The equivalent of a diffusion layer is then created, but inside the same molecule.
Fundings
ANR FastGiant (2017-2021, N° ANR-17-CE07-0012-01)
Publications récentes
- Zhou, X. S., Mao, B. W., Amatore, C., Compton, R. G., Marignier, J. L., Mostafavi, M., Nierengarten, J. F., and Maisonhaute, E. (2016) Transient electrochemistry: beyond simply temporal resolution, Chem. Commun. 52, 251-263.
- Fortgang, P., Urbani, M., Holler, M., Nierengarten, J.-F., Moreau, A., Delavaux-Nicot, B., and Maisonhaute, E. (2015) Electron Transfer Rates in an Adsorbed C60-Porphyrin Dyad, Electroanalysis 27, 1010-1016.
- Zhou, X. S., Liu, L., Fortgang, P., Lefevre, A. S., Serra-Muns, A., Raouafi, N., Amatore, C., Mao, B. W., Maisonhaute, E., and Schollhorn, B. (2011) Do Molecular Conductances Correlate with Electrochemical Rate Constants? Experimental Insights, J. Am. Chem. Soc. 133, 7509-7516.
- Fortgang, P., Maisonhaute, E., Amatore, C., Delavaux-Nicot, B., Iehl, J., and Nierengarten, J. F. (2011) Molecular Motion Inside an Adsorbed 5:1 Fullerene Hexaadduct Observed by Ultrafast Cyclic Voltammetry, Angew. Chem. Int. Ed. 50, 2364-2367.